BREAKTHROUGH VICTORIA PORTFOLIO COMPANY NAVI MEDICAL TECHNOLOGIES REACHES MAJOR MILESTONE
Breakthrough Victoria portfolio company, Navi Medical Technologies, has taken a major step in their mission to making hospital care safer for newborn babies and children.
Navi’s world leading technology, the Neonav® ECG Tip Location System, has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). This achievement not only validates the company’s groundbreaking technology but also positions Navi for significant growth in the U.S. healthcare market.
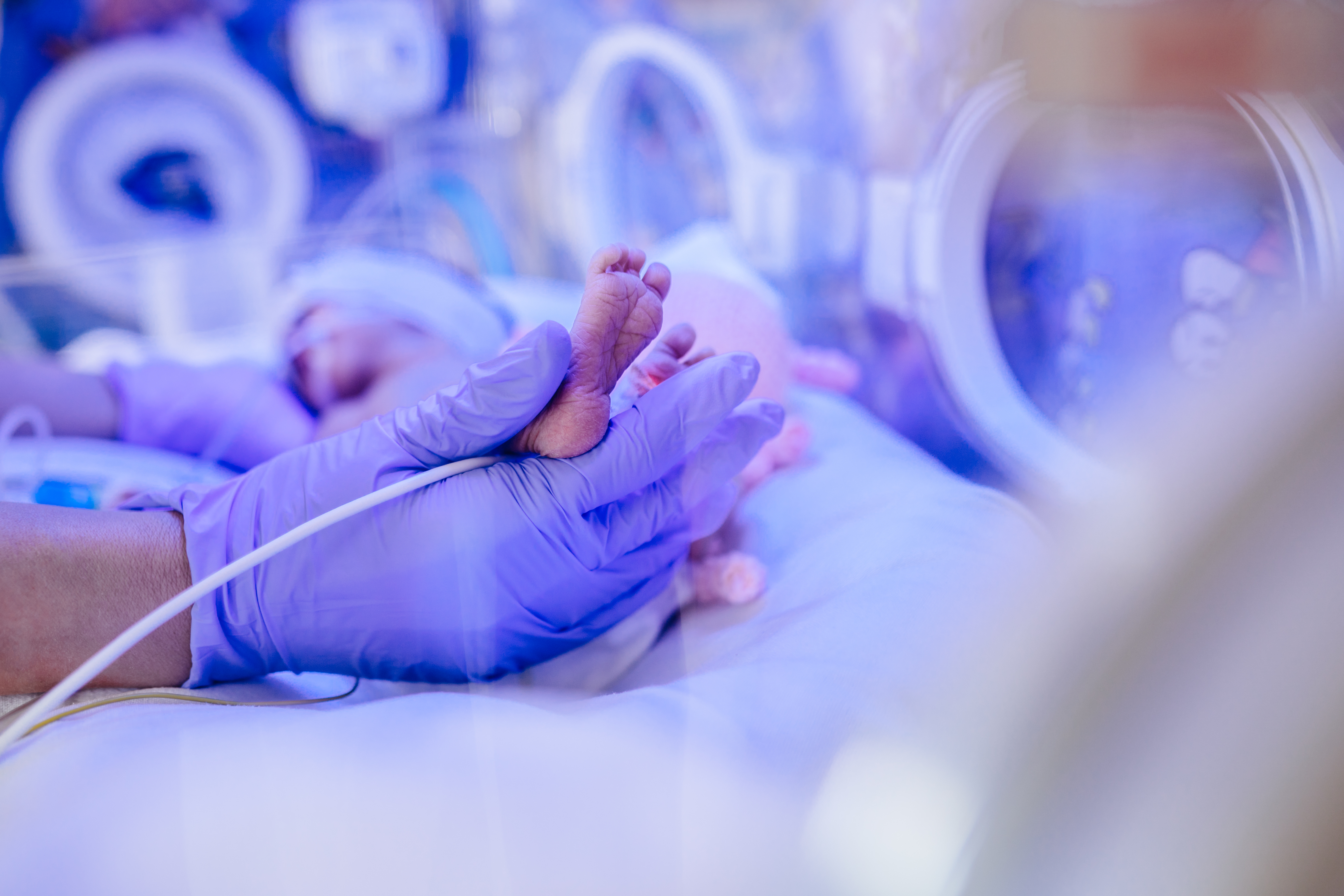
The Melbourne startups medical device helps guide clinicians inserting central venous catheters into the veins of critically ill newborn babies and children to administer life-saving therapies.
Currently, almost half of these life-saving procedures result in catheters being misplaced, which if left undetected, can lead to life threatening complications. The high misplacement rate is due to clinicians relying on x-rays to confirm the catheter location, which can only be done after the procedure has taken place.
The Neonav® system is the first of its kind specifically designed to improve vascular access in critically ill newborns and children. By leveraging real-time ECG signal analysis, it enables precise placement of Central Venous Access Devices (CVADs), reducing risks associated with misplacement and migration—issues that can cost issues that can cost hospitals billions annually
Additionally, Neonav® helps minimise reliance on chest X-rays, improving efficiency and reducing complications for vulnerable paediatric patients.
The addressable market for this technology is vast, and Navi is strategically positioned to capture significant market share in the U.S. and beyond.
Following this regulatory success, Navi plans to raise capital later this year to support commercial rollout in the U.S. healthcare system, with plans to secure Australian and European regulatory clearance and replicate the rollout locally and across the rest of the globe.
This milestone is a testament to the strength of Navi’s leadership team, the quality of its innovation, and the unwavering support of investors and partners, including Breakthrough Victoria, The Royal Women’s Hospital, the Australian and Victorian Governments, MTPConnect, and the US FDA Paediatric Device Consortia program.
Quote attributable to Breakthrough Victoria CEO Rod Bristow.
“As an investor, we are excited about Navi’s trajectory and its potential to become a leader in the paediatric medical device market. With strong fundamentals, regulatory validation, and a clear pathway to commercialisation, Navi represents a compelling investment opportunity with both financial and wider societal impact."
Quote attributable to Navi Technologies CEO and co-founder Alex Newton
“With FDA clearance in hand, we have a unique opportunity to drive substantial impact in one of the world’s largest healthcare markets. For our investors, this represents not just a financial opportunity but a chance to be part of a company dedicated to saving lives and improving pediatric healthcare on a global scale.”
Quotes attributable to Minister for Economic Growth and Jobs Danny Pearson
“This local Victorian company will be taking its home-grown technology to the United States - helping make hospital care safer for critically ill newborn babies on the other side of the world.”
“Victoria is the medical research capital of Australia – this is a booming industry that is backing economic growth and quality advanced manufacturing jobs across our state.”
Quotes attributable to Dr Janene Feurch, Neonatologist at Stanford Children’s Health
“Central lines are lifesaving but can be challenging to position. We need a guidance system to tell us when we are in the correct location. The Neonav system fulfills that need and gives a characteristically blind procedure a way to move forward, telling you exactly where you are and how to move into the correct position”